The elusive THRIP
Vegetable growers need to stay vigilant for chilli thrips – this tiny pest can create big problems if left unmanaged, and it won’t be content to stay within current crops.
Words Helen Newman and Aileen Reid, APC
CHILLI thrips have quickly jumped onto the radar of many horticulturalists in Western Australia. This is despite this pest’s presence in northern Australia for about 20 years. The first complaints from home gardeners in the Perth metro area were received in 2020. This was followed by table grape growers the following year, by berry growers in 2021/22 and it has now spread to capsicums, chillis and tomatoes. However, don’t underestimate this pest. It has a wide host range of 225 plant species, is known around the world to be notoriously difficult to manage and can cause significant economic damage to horticulture. With this in mind, WA growers are encouraged be vigilant and keep up to date with monitoring and control recommendations.
So where did they come from? Chilli thrips (S. dorsalis), also known as strawberry and yellow tea thrips, are native to Asia. They were first recorded in the US in Florida in 1991 and established there in around 2005. Since then, they have become a major pest for many horticultural crops. They have also been reported in various parts of Africa and are established in Israel, the Solomon Islands, the Caribbean and South America.
Sap-sucking insects, these pests cause deformities in flowers, leaves, stems and shoots. They are also a known vector of several viruses, including tomato spotted wilt virus and tobacco streak virus. In WA, while it has been reported on table grapes, strawberries, blueberries, blackberries, capsicums, chillis, roses and tomatoes, there are many other hosts reported, including a wider range of fruit and vegetable crops. Nightshades and acacia are also listed as hosts and may be responsible for overwintering.
Identification
This type of thrip is pale yellow. It’s very small – 0.5-1.2mm long – and hard to distinguish from other species without a microscope. Key distinguishing features are eight segmented antennae, red ocelli and two pairs of bristles below the prothorax (see Figure 1).
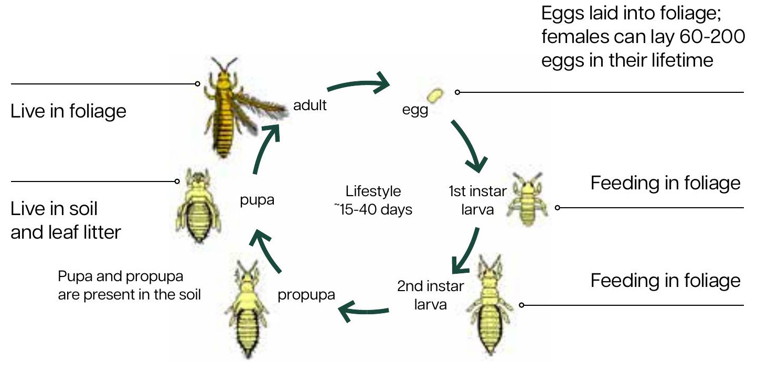
Figure 2: Chilli thrips’ lifecycle lasts from 15-40 days depending on temperature and host plant.
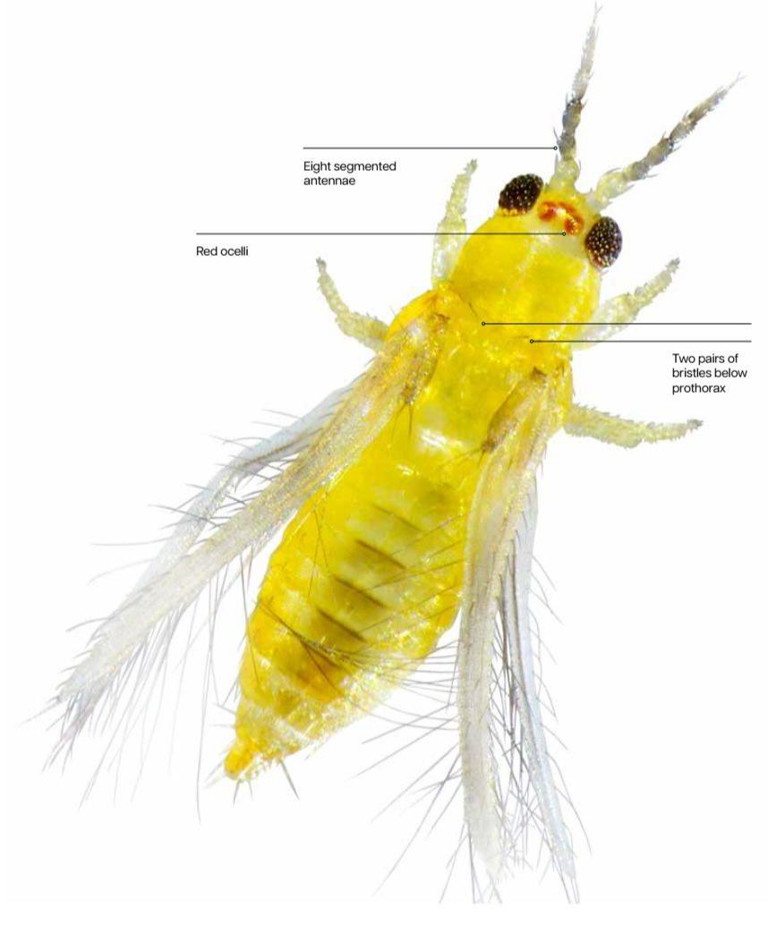
Figure 1: Distinguishing features of Chilli thrips. Red ocelli
Its lifecycle, comprising egg, larva, pre-pupa, pupa and adult (Figure 2), lasts from 15-40 days, depending on temperature and host plant. For example, it takes 11 days to become an adult from first instar larva on capsicum plants and 13 days on squash at 28°C. Adults can survive for 15 days on eggplant, but 13 days on tomato plants and roses. They can survive at minimum temperatures as low as 9.7°C and maximum temperatures as high as 33°C. They can have many generations in a single year and populations can build up quickly over a short time. In the Perth region, up to 13 generations may be possible (Figure 3). This would likely be higher for those under high tunnels and in greenhouses. Understanding the lifecycle of the pest will help with management options and schedules.
“ … don’t underestimate this pest. It has a wide host range of 225 plant species ... and can cause significant economic damage to horticulture ”
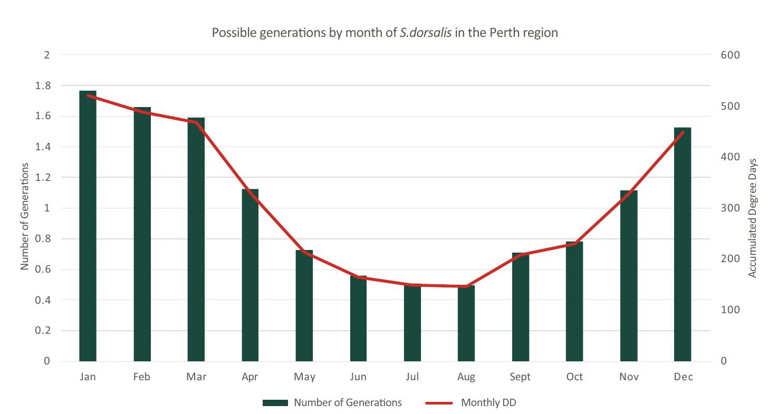
Figure 3: Possible generations by month of chill thrips in Perth. Data courtesy Elliot Howse, DPIRD
In terms of seasons, they are most active during spring to autumn. In Western Australia, dryness and higher temperatures create conditions conducive to chilli thrips feeding, spread and reproduction. Like most thrips, they feed on tender plant parts, which result in undesirable feeding scars, distortion of leaves and discoloration of buds, flowers and young fruits. It doesn’t feed on mature host tissues.
So how do we control it?
Chilli thrips can quickly develop resistance to chemicals, so biological controls are important in a management strategy. Currently, cucumeris mites and orius bugs are being used in local IPM strategies and their rates of application are adjusted according to pest pressure.
Cucumeris feed on small (1st and 2nd instar) thrips on foliage and flowers, but will not feed on large larvae or adult thrips. They are a generalist predator and will also feed on other mites. Cucumeris take 8-11 days at (25-20°C) to grow from an egg to adult and they can live up to 32 days. Females produce an average of 35 eggs in their lifetime. Cucumeris prefer environments with greater than 65 per cent relative humidity (RH), but eggs can survive as low as 40 per cent RH.
All life stages of orius feed on live prey and adults can kill up to 20 thrips a day. When pest populations are high, orius will kill more thrips than are required for their nutritional needs. Orius adults can fly and move through a crop quickly when searching for food.
The development time from egg to adult can vary from 16-18 days at 25°C, to 12 days at 30°C. Females lay at a relatively slow rate of around 2-3 eggs per day, but adults live for 3-4 weeks.
Identify Damage


Upward rolling of leaves, deformed leaves and fruit, and zipper-like scarring is seen on capsicum plants.
Photography: Cezar Moraes
If you see these issues, the pest is already well established in the crop.
Chilli thrips have piercing and sucking mouthparts, and cause damage by extracting the contents of individual epidermal cells, which leads to damaging feeding scars (usually silver, brown or black), distortion of leaves and discoloration of buds, flowers and young fruits. Upward rolling of leaves (referred to as ‘chilli leaf curl’ in capsicums) and reduced leaf size are also common. A severe infestation of chilli thrips can make tender leaves and buds brittle, resulting in complete defoliation and total crop loss. Infested soft fruits can develop corky tissues, sometimes with zipper-like scarring on larger fruits, such as capsicum. On citrus, chilli thrip damage can appear as bronzing and silvering on the rind. Chilli thrip infestations are not always easy to identify and can sometimes be mistaken as other pest problems, such as broad mite, so monitoring is important.

Figure 4: Blue and yellow sticky traps used in monitoring and management trials in local berry crops. Photography: Cezar Moraes

“ Chilli thrips can quickly develop resistance to chemicals, so biological controls are important ”
In terms of chemical control, the insects can move to surrounding weed crops to take refuge and they lay eggs inside the leaf tissue (in leaf curls or under fruit calyxes) where they are protected from pesticide sprays. Trials by Biological Services in WA used a range of pesticides. Chemicals that offered temporary reductions in numbers were:
• Entrust™ (Spinosad – Group 5 insecticide)
• Hymal™ or Fyfanon™ (maldison – Group 1B insecticide)
• Benevia™ (Cyantraniliprole – Group 28 insecticide)
Control was improved when adjuvants were used. However, always check the label and the APVMA website before applying a chemical. It was also important to maintain a weekly monitoring schedule from spring to autumn, more selective chemicals and apply insecticides in rotation and avoid using the same chemical more than three times during the season to help prevent resistance.
So, what further work is needed to combat the establishment of chilli thrips? There is a need to develop a better understanding of this pest’s biology across growing environments and hosts. We know they are prone to developing insecticide resistance, so it is important to develop an understanding of how they interact with chemistries. To combat insecticide resistance, further work is needed to develop biological control agents either by optimisation of currently available biological controls or investigating new opportunities. In the meantime, stay vigilant.
MORE INFORMATION
For help in managing chilli thrips, contact Biological Services’ Muchea office on 9571 4849, info@ biologicalservices.com.au. This study was made possible thanks to the Australian Berry Industries MT22010 project. Thank you to Cezar Moraes of Biological Services and Elliot Howse of DPIRD’s Insect and Disease Management department for contributing to the writing of this article.
Four Steps to Monitoring Thrips
Early detection and control of thrips before populations build in essential.
1 Yellowsticky traps are most commonly used and are effective; other research shows that blue can also be effective (Figure 4).
2 Cropscouting includes direct methods, such as counting thrips on a host plant (eg leaf, flower. fruit) using a (10x) hand lens, microscope or the naked eye.
3 Tappingflowers or branches into a white paper board will dislodge adults for counting.
4 Samplingmethods must take account of the distribution of the thrips within the crop as the pest is often unevenly distributed.