BEEINFORMED
INTEGRATING VARROA SENSITIVE HYGIENE
into Western Australian queen breeding programmes
As Varroa destructor continues its spread across eastern Australia, the apiculture industry in Western Australia (WA) is proactively exploring genetic resistance strategies — particularly the integration of Varroa Sensitive Hygiene (VSH) traits into local honey bee (Apis mellifera) queen breeding programmes. The current focus is on actions that must be taken before Varroa’s arrival to reduce its eventual impact.
Words Gavin Phillips, WA Pure Honey
The Tocal Workshop: a national breeding collaboration
IN May, a cohort of WA queen breeders participated in a national VSH breeding workshop hosted at the Tocal Bee Research Centre in New South Wales. Led by NSW DPI technical specialist Elizabeth Frost, the workshop brought together around 30 breeders, researchers, and extension officers from across Australia, with remote support from VSH specialists in the United States.
The multi-day training covered practical field and laboratory techniques for identifying and quantifying VSH expression, including hygienic response assays, mite population monitoring, and data collection strategies.
Participants also engaged in in-depth discussions on honey bee genetics, artificial insemination (AI), and the use of data recording tools for genetic evaluation and traceability.

Understanding VSH: resistance mechanisms detectable before varroa arrives
Varroa Sensitive Hygiene is a heritable behavioural trait in which bees detect and disrupt the reproductive cycle of Varroa destructor. Bees exhibiting VSH behaviour typically uncap and remove pupae infested with reproducing mites, thereby reducing mite reproduction rates and slowing population growth within the colony.
Other resistance-associated behaviours include grooming and mite biting, though VSH is distinct in its focus on mite-infested brood cells.
Crucially, while all VSH bees demonstrate high levels of general hygienic behaviour, not all hygienic bees express VSH, necessitating specific assays to differentiate the two traits.
Observations from commercial operations
The workshop included site visits to several major beekeeping enterprises affected by Varroa, including the Lockwood operation, which manages over 16,000 hives and nucleus colonies. Despite being 18 months post-infestation, the Lockwood colonies showed robust health indicators, even during cold-weather inspections.
However, maintaining hive health has required substantial chemical interventions and increased labour capacity. The operation has employed a full-time specialist queen breeder to conduct comparative trials of treated and untreated colonies, as well as artificially inseminated queens selected for resistance traits.
A separate visit to veteran queen breeder Joe Horner and his family team showcased alternative breeding strategies, including a custom-built rail system for controlled mating timing. Horner’s meticulous genetic selection, supported by decades of phenotypic and colony-level data, underscores the critical role of record keeping in developing resistance traits.
Real-world limitations of VSH
While VSH shows promise as a resistance mechanism, current research and field data suggest it is not sufficient on its own to control Varroa destructor under high-pressure conditions. Colonies expressing VSH traits can maintain mite loads at manageable levels (10–15 mites) in the absence of reinfestation. However, the collapse of nearby untreated colonies, so-called “mite bombs”, can rapidly increase mite loads across neighbouring hives by 50–100+ mites, often overwhelming even VSH colonies.

Notably, eastern Australian apiaries have observed colony survival at mite counts well over 300, a scenario rarely seen overseas. This phenomenon is likely due to the current absence of several Varroa-vectored viruses, such as Deformed Wing Virus, which exacerbate colony morbidity in other regions.
The importation paradox
Importing resistant stock from overseas is currently neither feasible nor legal, for several key reasons:
1. Adaptability: Bees bred for resistance in temperate or tropical climates often perform poorly in WA’s Mediterranean environment. WA bees trialled in cooler climates have shown poor overwintering due to excessive brood rearing and insufficient resource conservation. Importing semen could also dilute the highly productive traits we’ve worked hard to establish in WA breeding genetics.
2. Biosecurity risks: Importation increases the risk of introducing exotic pathogens, particularly Deformed Wing Virus (DWV) and other Varroa-associated viruses, which are currently absent in Australia.
3. Limited initial resistance: Even highly resistant stock requires treatment during initial infestation waves. The myth of a “magic bullet” resistance trait has been debunked by field trials globally.
The case for pre-emptive breeding in WA
Given that initial infestation waves are likely to result in extreme mite loads (500–1,000 mites per colony were recorded during early eradication efforts in NSW), pre-emptive breeding for resistance is essential.
Waiting to select survivors’ post-infestation is not viable, as even genetically resistant colonies cannot tolerate such acute infestations without intervention.
In fact, failure to treat may result in the loss of genetically valuable stock. This highlights the urgency of identifying and preserving VSH-positive lines before Varroa becomes established in WA.
Next steps for WA breeders
Adapting WA breeding programmes to search for VSH traits prior to Varroa’s arrival will require a phased approach. Once breeders have completed their current assessments to select next season’s breeding stock, those displaying desirable traits for WA conditions can be further evaluated for VSH using the following steps:
1. Baseline hygienic testing
Initial screenings using established methods (e.g., pin-kill or freeze-kill assays) will identify colonies with high general hygienic behaviour (see Figure 1).
2. VSH identification and genetic recording
Colonies exhibiting strong general hygienic traits will undergo targeted VSH screening, supported by specialised evaluation tools such as UbeeO.
3. Artificial insemination and controlled trials
Selected queens will be inseminated and assessed for VSH expression. Successful candidates can then be sent to Varroa-infested regions for further evaluation in WA-like environments.
4. Continual Breeding Programmes
Once Varroa arrives in WA, more accurate Varroaspecific assays (e.g., Harbo Assay) can be used to further refine resistance traits.

Hygienic behaviour Liquid nitrogen freeze test.
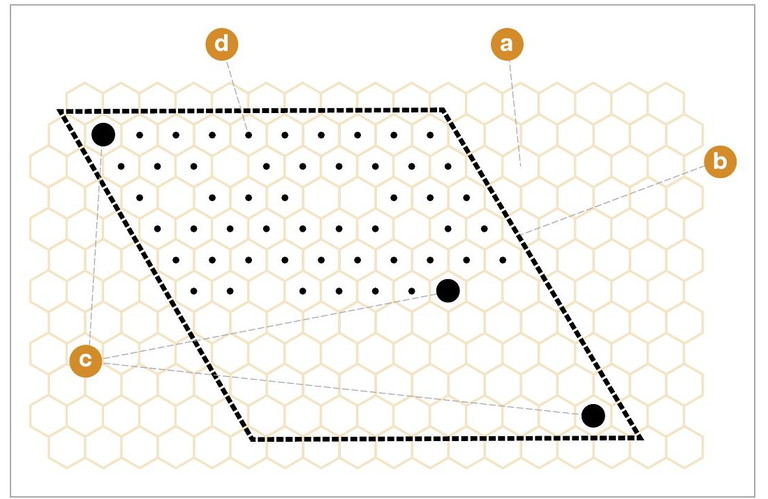
FIGURE 1. Pin Kill Test
1. A rhomboid frame of a 10 × 10 cell wide template (b) is placed on a brood comb containing young pupae (a).
2. The upper left and lower right cells are marked with a colour felt-tip pen (c).
3. 50 capped brood cells are pierced (d) row by row from left to right with a fine insect pin (entomological pin size No 2).
4. Cell 51 is marked with a colour felt-tip pen to identify the treated brood area (c).
5. The comb is marked on the top bar and placed back to the brood nest in its former position.
6. After 6h, the removal progress is checked. All cells that are still sealed are counted and subtracted from 50.
Source: Standard methods for rearing and selection of Apis mellifera queens 2.0
A call for collective action
Developing VSH breeding capacity in WA is both strategically and financially essential. While the trait alone cannot eliminate Varroa-related colony losses, it is a vital component of integrated pest management strategies. Long-term sustainability will depend on a combination of selective breeding, rigorous data collection, and industry-wide collaboration.
WA now has the opportunity to learn from the eastern states and begin laying the genetic and institutional groundwork, before Varroa arrives.
MORE INFORMATION
Gavin Phillips, gavin@wapurehoney.com


Scan me